Last week we had our annual first-year 'paramecium practical'. The aims of the session were to familiarise the students with the microscopes and to also introduce unicellular organisms.
In the UK we order the paramecium for the practical from a local supplier. However, in Malaysia, despite 4 months of searching, I was unable to find a supplier. This left me with three options:
- Don't do the practical
- Find an alternative supplier
- Culture my own…
I tried to find an alternative supplier (paramecium are used to feed baby fish), but none of the local pet stores stocked them - although I did get a rather nice bag of water fleas (Daphnia).

A bag of Water Flea - Daphnia

A Water Flea - Daphnia
Finally, I decided to culture them.
After a quick read around the Internet, I found a recipe for culture media and some advice on where to find paramecium.
The general advice for 'hunting' paramecium is to take water from close to the top or the edge of a pond. In the end, I took a sample of water from the surface, plus some material from the bottom of the pond. This was allowed to settle overnight (see photo below).
The culture media consisted of dog biscuits, and 4 or 5 cm2 of lettuce leaf, in water.
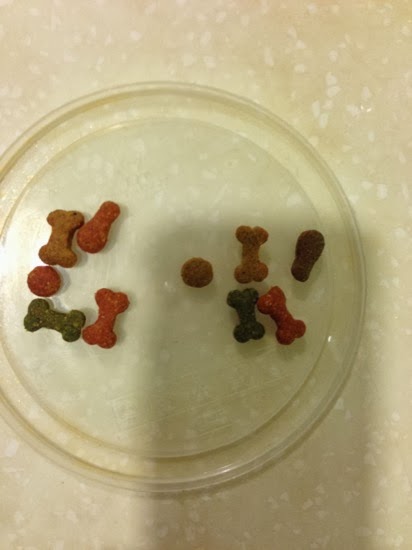
Dog Biscuits

Lettuce Leaf

Water

Pond Water - a mixture of material from the bottom of the pond, and water from the surface and edge

Two culture bottles and a bottle of bond water
I set up three bottles, two culture media, and a bottle of pond water. These were left overnight.
And the next morning… The pond water had cleared, and the bottles with dog biscuits and lettuce were cloudy (the cloudiness was caused by bacteria growing) - these will be a food source for the paramecium.
One of the 'culture media' bottles was seeded with 20 ml of water from the top of the pond bottle, and the other with 20 ml of water collected near the vegetation at the bottom of the pond bottle.

Next morning - the three bottles…..
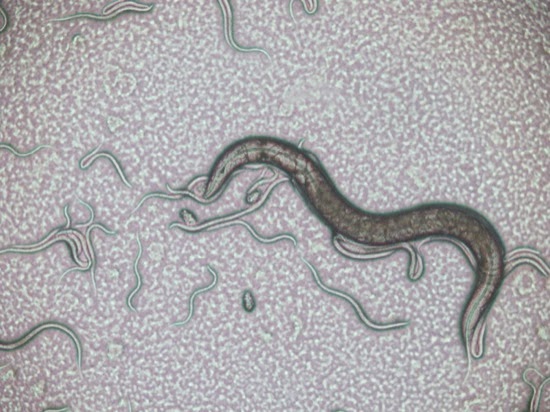
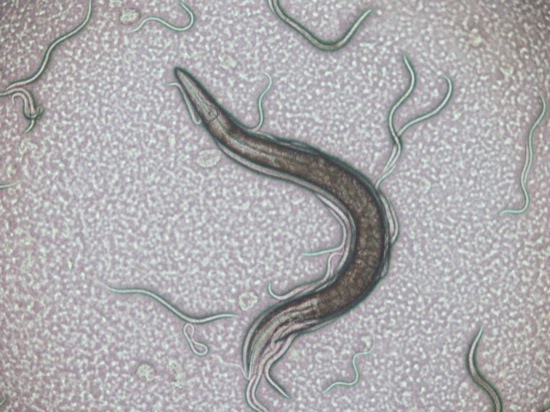
An examination of the pond water under a microscope revealed the presence of paramecium, but not that many.

There were also some other ciliates present…

Over the next 12 days, the cultures got progressively more cloudy and exceedingly smelly.

12 days after adding the pond water
Of the two culture bottles, the one seeded with water from the top of the pond bottle produced the best (most and largest) paramecium, and the best place to harvest the paramecium was from the top 5 - 10 mm of the bottle, and in particular the layer of 'scum' that had formed on the top of the water. Paramecium taken from elsewhere in the bottle tended to be smaller.
Movie
The movie below was shot during the practical.
If you would like to support my blogging efforts, then please feel free to buy me a coffee at https://www.buymeacoffee.com/drnickm