This one causes students problems every year - the relationship between Glycogen Synthase Kinase 3 (GSK3) and Glycogen Synthase (GS), and their phosphorylation states and activity.
If you look at insulin signalling overall:
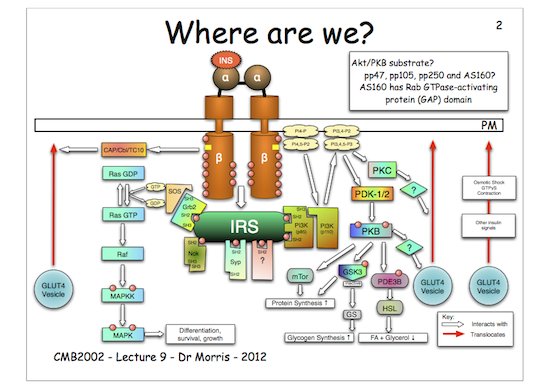
You will see that activated protein kinase B (activated by phosphorylation) phosphorylates and inactivates GSK3. This means that GSK3 can no longer phosphorylate its substrate, GS.
Now, GS, when phosphorylated, is inactive. Hence, as GSK3 is no longer active (as it is phosphorylated) it cannot phosphorylate GS and inactivate it, therefore GS remains un-phosphorylated and therefore active so it can make glycogen.

The key to understanding this is that phosphorylated (inactive) and un-phosphorylated (active) forms of GS are in a state of equilibrium and that the kinase, GSK3, drives the equilibrium to the right (inactive) in the figure below, whereas the phosphatase (which removes the phosphate) drives the equilibrium to the left (active). Therefore, if you inhibit the action of the kinase (GSK3) the net result is more un-phosphorylated GS, hence more glycogen is made, which is just what we want…
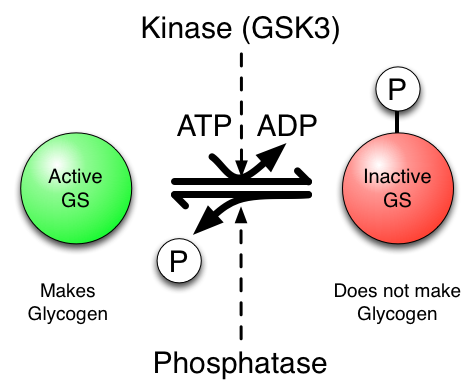
One key thing to keep in mind is that the phosphorylation of a protein can cause it to become active or inactive. It all depends on the protein.
If you would like to support my blogging efforts, then please feel free to buy me a coffee at https://www.buymeacoffee.com/drnickm
Additional Resources
- 📗 - Biochemistry (Stryer) (affiliate link)
- 📗 - Principles of Biochemistry (Lehninger) (affiliate link)
No comments:
Post a Comment